Use a new disposable PODHALER device and storage case every 7 days. The PODHALER device should always be stored in its case and tightly closed when not in use.1
TOBI PODHALER Supply and Storage
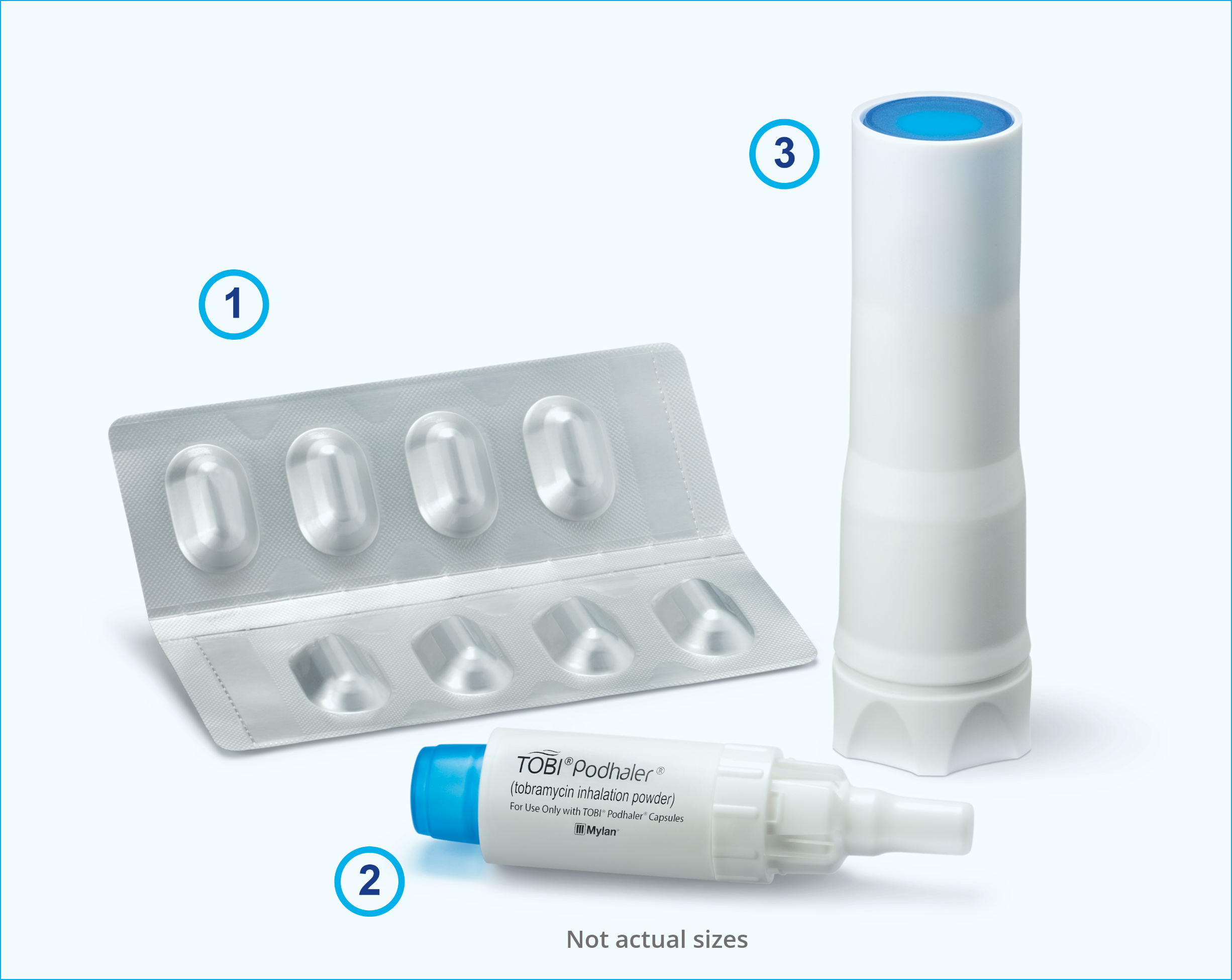
TOBI PODHALER comes in a 28-day multipack that has everything you need for 1 treatment cycle.1
- 4 weekly packs, each containing 56 capsules (7 blister cards of 8 capsules)
- 4 disposable PODHALER devices plus 1 backup PODHALER device
- 4 storage cases plus 1 backup
Do…
Wipe the mouthpiece with a clean, dry cloth
Store in a dry place at room temperature (≈77°F); excursions permitted to 59°F to 86°F
Protect TOBI PODHALER from moisture
- TOBI PODHALER capsules should be used with the PODHALER device only
The PODHALER device should not be used with any other capsules - Store TOBI PODHALER capsules in the blister card; each capsule should only be removed
IMMEDIATELY BEFORE USE - Always use the new PODHALER device provided with each weekly pack
Reference
- TOBI® PODHALER® [Prescribing Information, 2023].

 MORE +
LESS –
MORE +
LESS –
INDICATION
TOBI® PODHALER® (Tobramycin Inhalation Powder) 28 mg per capsule is indicated for the management of cystic fibrosis patients with Pseudomonas aeruginosa.
Safety and efficacy have not been demonstrated in patients under the age of 6 years, patients with forced expiratory volume in 1 second (FEV1) <25% or >80% predicted, or patients colonized with Burkholderia cepacia.
IMPORTANT SAFETY INFORMATION
TOBI PODHALER is contraindicated in patients with known hypersensitivity to any aminoglycoside.
Bronchospasm can occur with inhalation of TOBI PODHALER. Bronchospasm should be treated as medically appropriate.
Caution should be exercised when prescribing TOBI PODHALER to patients with known or suspected auditory, vestibular, renal, or neuromuscular dysfunction.
Ototoxicity, as measured by complaints of hearing loss or tinnitus, was reported by patients in the TOBI PODHALER clinical studies. Tinnitus may be a sentinel symptom of ototoxicity, and therefore the onset of this symptom warrants caution. Ototoxicity, manifested as both auditory and vestibular toxicity, has been reported with parenteral aminoglycosides. Vestibular toxicity may be manifested by vertigo, ataxia, or dizziness.
Cases of ototoxicity with aminoglycosides have been observed in patients with certain variants in the mitochondrially encoded 12S rRNA gene (MT-RNR1), particularly the m.1555A>G variant. Ototoxicity occurred in some patients even when their aminoglycoside serum levels were within the recommended range. Mitochondrial DNA variants are present in less than 1% of the general US population, and the proportion of the variant carriers who may develop ototoxicity as well as the severity of ototoxicity is unknown. In case of known maternal history of ototoxicity due to aminoglycoside use or a known mitochondrial DNA variant in the patient, consider alternative treatments other than aminoglycosides unless the increased risk of permanent hearing loss is outweighed by the severity of infection and lack of safe and effective alternative therapies.
Caution should be exercised when prescribing TOBI PODHALER to patients with known or suspected renal dysfunction. Nephrotoxicity was not observed during TOBI PODHALER clinical studies but has been associated with aminoglycosides as a class.
TOBI PODHALER should be used cautiously in patients with neuromuscular disorders, such as myasthenia gravis or Parkinson’s disease, since aminoglycosides may aggravate muscle weakness because of a potential curare-like effect on neuromuscular function.
Aminoglycosides can cause fetal harm when administered to a pregnant woman. Patients who use TOBI PODHALER during pregnancy, or who become pregnant while taking TOBI PODHALER, should be apprised of the potential hazard to the fetus. The amount of tobramycin excreted in human breast milk is unknown. However, systemic absorption of tobramycin following inhaled administration is expected to be minimal. A decision should be made whether to discontinue nursing or TOBI PODHALER. TOBI may cause intestinal flora alteration. Advise a woman to monitor the breastfed infant for loose or bloody stools and candidiasis.
Patients receiving concomitant TOBI and parenteral aminoglycoside therapy should be monitored as clinically appropriate for toxicities associated with aminoglycosides as a class. Serum tobramycin levels should be monitored.
Concurrent and/or sequential use of TOBI PODHALER with other drugs with neurotoxic, nephrotoxic, or ototoxic potential should be avoided. Some diuretics can enhance aminoglycoside toxicity by altering antibiotic concentrations in serum and tissue. TOBI PODHALER should not be administered concomitantly with ethacrynic acid, furosemide, urea, or mannitol.
In a clinical trial, the most commonly observed adverse events with TOBI PODHALER occurring at a frequency of at least 10%, were cough, lung disorder, productive cough, dyspnea, pyrexia, oropharyngeal pain, dysphonia, hemoptysis, and headache.
Please see Full Prescribing Information

